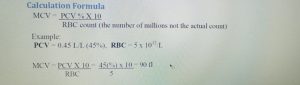Abstract
Blood smears are made to give an overview picture of the number, cell shape, size of blood cells. Blood and bone marrow films that are well prepared and properly stained have a great value in haematology. They are used in:
- Viewing cell morphology for diagnosing anemias and leukemias,
- WBC differential counts
- Estimating the number of platelets.
Technique of Spreading
Spreading is done on dust free glass microscope slides using a spreader, which is a microscope slide having one corner removed at each end.
Types of Blood smears
- Thick blood smears which are used for parasitology e.g. malaria is prepared by handling the spreader by the edge, using the corner to spread the blood in a circular form with 3-6 movements.
- Thin blood smears which are used in hematology and prepared as below
Preparation of thin blood smears
A good thin smear has blood spread in a layer such that the thickness decreases progressively toward the feathered edge.
In the feathered edge, the cells appear in a monolayer, not touching one another.
EDTA anti-coagulated blood is used. Blood is taken using capillary tubes.
- Place a drop of blood near the end of the slide, about 1-2 cm from one end.
- Place the spreading slide at an angle of 45◦ about 1 cm in front of the drop of blood.
- Back the spreader into the drop of blood. The spreader catches the blood and it spreads by capillary action along its edge.
- Maintaining the 45◦ angle, push the spreader smoothly across the slide.
- This pulls the blood across to make the smear.
- It should take about one second to make the smear.
- Allow the film to air dry.
Notes on method:
- Make at least 2 smears for every patient
- The edge of the spreader must be very smooth, and narrower than that of the slide.
- The spreader must be cleaned and dried if it had been used for spreading more than five films.
- A smooth action is required, with the edge of the spreader held against the slide.
- Films may be prepared manually or automated slide spreaders.
- The blood film length shouldn’t be too long nor should it be too short.
- All slides should be labeled for identification.
- In most haematology laboratories today, automated blood film makers and strainers are used. Studies are still being conducted to compare the slides made by automated slide makers to those that are made manually. The manufacturer’s instructions should be followed unless local experience has demonstrated that variation of the recommended technique achieves better results.
Thick blood smears
Thick smears usually have a thick layer of dehemoglobinized (lysed) red blood cells (RBCs). The blood elements (including parasites, if any) are more concentrated than in an equal area of a thin smear. Thus, thick smears gives a more efficient detection of parasites (increased sensitivity). But, they do not permit an optimal review of parasite morphology. For example, they are often not enough for species identification of malaria parasites: if the thick smear is positive for malaria parasites, the thin smear is done for species identification.
Make at least 2 smears per patient
- Put a small drop of blood in the center of the pre-cleaned, labeled slide.
- Using the corner of another slide or an applicator stick, spread the drop in a circular pattern until it is the size of a dime (1.5 cm2) or button size.
- A thick smear of proper density is one which, if placed (wet) over newsprint, allows you to barely read the words.
- Lay the slides flat and allow the smear to dry properly (protected from dust and insects). Improperly dried smears (and/or smears that are too thick) can detach from the slides during staining. The risk is increased in smears made with anticoagulated blood. At room temperature, drying can take several hours; 30 minutes is the minimum; in the latter case, handle the smear very delicately during staining. Drying can be accelerated by using a fan or hair dryer (use cool setting).
Scratch Method for Thick blood smears
This method is an alternate for preparing thick films that allows for better adherence and faster turnaround times. The process is just similar to making a normal thick film, but instead of using a stick to spread the blood, the edge of a glass microscope slide is used, while applying firm pressure to create small scratches in the underlying slide. The scratches allow for improved adherence of the blood film to the slide without affecting the smear morphology. The smear can then be stained as soon as it is dry, generally within 20-30 minutes of smear preparation.
Notes on method:
- Protect thick smears from hot environments to prevent heat-fixing the smear.
- Do not fix thick smears with methanol or heat.
- If there will be a delay in staining smears, dip the thick smear briefly in water to haemolyse the RBCs.